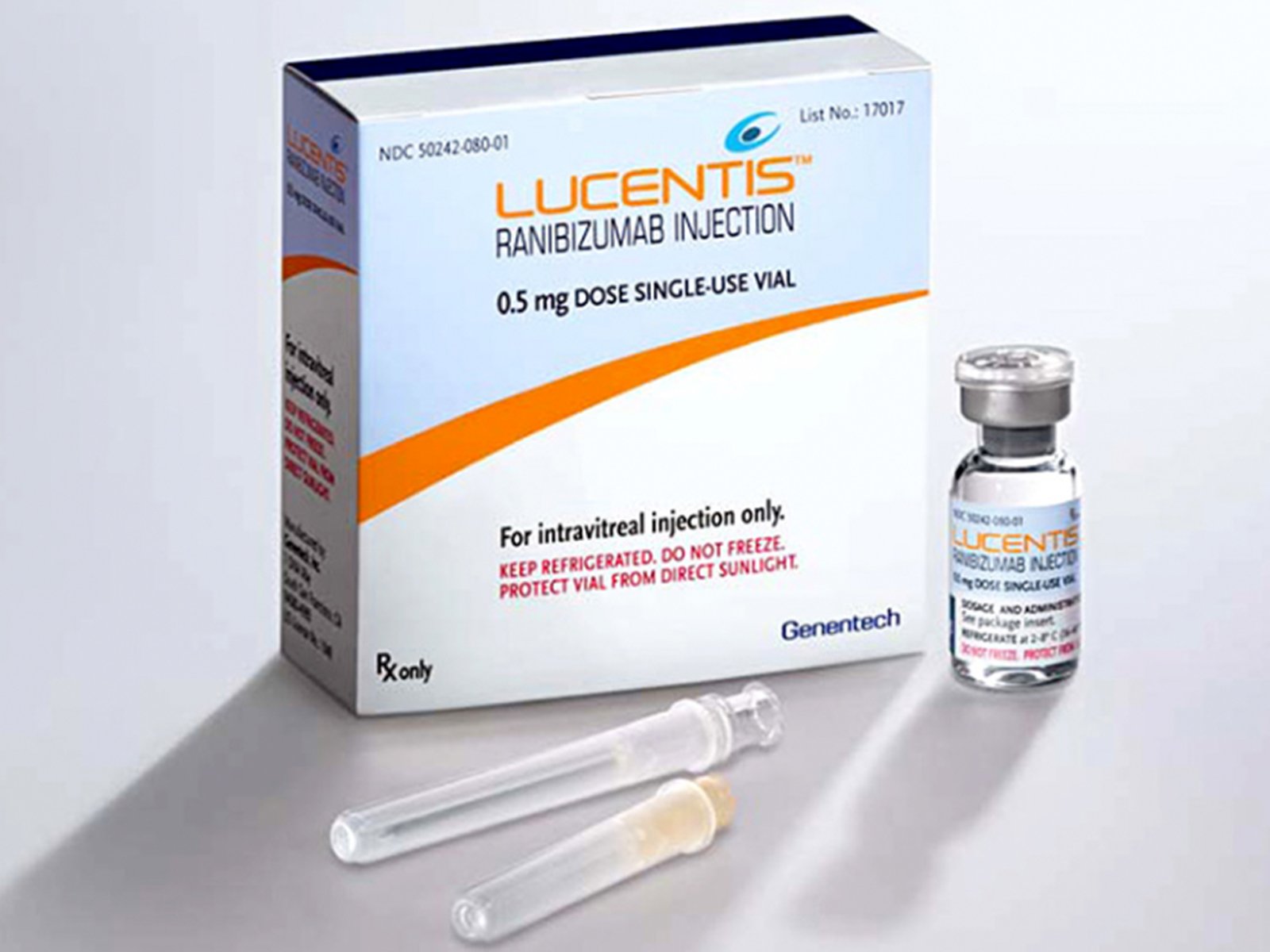
Lucentis ranibizumab Injection
Function:
For the treatment of wet (neovascular) age-related macular degeneration (AMD). For the treatment of visual impairment caused by macular edema secondary to retinal vein occlusion (RVO) (branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO)). For the treatment of visual impairment caused by choroidal neovascularization (CNV) secondary to pathological myopia (PM) and other causes.
Dosage:
Drug Dosage: This product is administered via intravitreal injection. The recommended dose is 0.5mg each time (equivalent to an injection volume of 0.05ml), administered once a month. If long-term monthly injection is not possible, it can also be administered once every three months after continuous monthly injection for the first three months. Compared with continuous monthly injections, during the initial 3 months, continuous monthly injections followed by 9 months of treatment, if administered every 3 months, the improvement in visual acuity will be approximately 5 letters less (ETDRS visual acuity) on average. or Snellen eye chart 1 line). During the treatment period, the patient’s vision changes should be monitored monthly. If significant vision loss occurs, further injection treatment with this product is required. The interval between injections should not be less than one month. Methods of Administration Before administering intravitreal injections, the patient’s past medical history should be thoroughly evaluated to assess the possibility of hypersensitivity reactions. Intravitreal injection of this product must be performed under sterile conditions, including the use of surgical hand disinfection, a sterile mask, sterile gloves, a sterile surgical drape, and a sterile eyelid opener (or similar instrument). The patient must be given an appropriate anesthetic and a topical ocular spectrum antibiotic prior to injection. Disinfect the periocular skin, eyelids, and eyeball surfaces before injection. Patients should be instructed to self-administer antibiotic eye drops 3 days before and after each injection, 4 times a day. The injection needle should be 3.5~4.0mm behind the corneoscleral limbus, aligned with the center of the eyeball, and inserted into the vitreous to avoid horizontal injection. Push the 0.05ml injection slowly, and be careful to change the scleral injection site during subsequent injections. Patients must be monitored for intraocular pressure and endophthalmitis after injection. Monitoring should include checking the blood perfusion of the optic nerve head immediately after injection, measuring intraocular pressure within 30 minutes, and performing ophthalmoscopy, slit lamp and fundus examination 2 to 7 days later. Patients should be instructed to report any symptoms of endophthalmitis immediately to their physician. Each injection is intended to treat only one eye as a single injection. If the fellow eye also requires treatment, a new bottle of injection solution must be used and the sterile field, syringe, gloves, surgical drape, eyelid opener, filter needle, and injection needle must be changed before injecting this product into the other eye. . The same medicines produced by different manufacturers may have inconsistent instructions. If you find any inconsistency in the instructions before taking the medicine, please consult your doctor or pharmacist in time.
Adverse reactions:
Adverse reactions are listed by system organ category and frequency. The frequency is as follows: very common (>1/10), common (>1/100 to <1/10), uncommon (>1/1000 to <1/100 ), rare (>1/10000 to <1/1000), very rare (<1/10000). Infections and contagions are common: Nasopharyngitis. Common: Influenza. Common diseases of the blood and lymphatic system: anemia. Nervous system disorders are common: headaches. Common: Stroke. Eye diseases are common: intraocular inflammation, vitritis, vitreous detachment, retinal hemorrhage, visual impairment, eye pain, vitreous floaters, conjunctival hemorrhage, eye irritation, foreign body sensation in the eye, increased tearing, prosthetic inflammation, dry eye , eye congestion, eye itching, and increased intraocular pressure. Common: retinal degeneration, retinal abnormalities, retinal detachment, retinal tear, retinal pigment epithelial detachment, retinal pigment epithelial tear, decreased vision, vitreous hemorrhage, vitreous abnormality, uveitis, iritis, iridocyclitis, cataract , posterior subcapsular cataract, posterior capsular opacification, punctate keratitis, corneal epithelial abrasion, anterior chamber flare, blurred vision, injection site bleeding, ocular hemorrhage, conjunctivitis, allergic conjunctivitis, ocular discharge, Flash hallucinations, photophobia, eye discomfort, eye and face pain, eye and face congestion, and conjunctival congestion. Uncommon: Hyphepyon, hyphema, keratopathy, iris synechiae, corneal deposits and corneal edema, corneal wrinkling, injection site pain/irritation, blindness, eyelid irritation. Respiratory, thoracic and mediastinal diseases are common: cough. Common gastrointestinal disorders: Nausea. Common skin and subcutaneous tissue disorders: allergic reactions such as rash, urticaria, pruritus and erythema. Musculoskeletal and connective tissue disorders are common: joint pain. Examination findings are common: elevated intraocular pressure.
Drug contraindications:
Disabled if allergic to this product
Share:
Products
Our offers
Health Classification
Let us work together to protect precious health