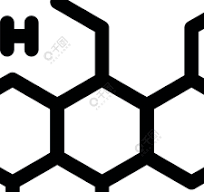
iptacopan Capsules Original drug Chinese packaging
On June 18, 2024, Ferheda® (iptacopan hydrochloride capsules) were gradually put on sale in China. Iptacopam is the world’s first specific oral inhibitor of complement factor B, which acts on the proximal pathway of the immune system’s complement bypass pathway, can fully control intravascular hemolysis and extravascular hemolysis, make up for the defects of anti-C5 antibody treatment, and is used to treat adult patients with paroxysmal nocturnal hemoglobinuria (PNH) who have not received complement inhibitor treatment before.
Recently, Novartis’ oral monotherapy, iptacopan hydrochloride capsules (iptacopan), was approved for marketing by the China National Medical Products Administration (NMPA) for the treatment of adult patients with paroxysmal nocturnal hemoglobinuria (PNH) who have not received complement inhibitor treatment before. Public information shows that this is a “first-in-class” complement pathway factor B inhibitor and the first oral monotherapy approved by the US FDA for the treatment of adult PNH. It was listed by industry media Evaluate as one of the 10 potential blockbuster therapies worthy of attention.
The approval of Iplocopam in China has also attracted people’s attention to PNH. PNH is a chronic, progressive, debilitating, life-threatening disease that has been included in China’s “First List of Rare Diseases”. The disease often occurs in young and middle-aged people, accounting for about 77% of those aged 20 to 40. Studies have found that the 5-year mortality rate of PNH patients is as high as 35%, and the median survival period is about 10 years [1]. The arrival of Iplocopam provides a new type of targeted treatment option for PNH patients. In today’s article, the WuXi AppTec content team will introduce the century-long history of mankind’s efforts to tackle PNH disease based on public information.
Share:
Products
Our offers
Health Classification
Let us work together to protect precious health