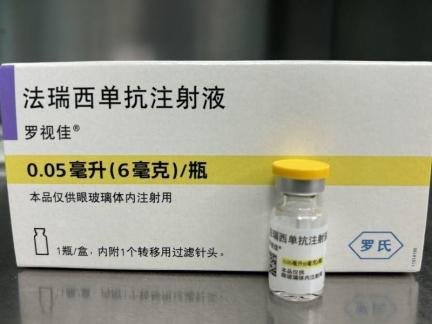
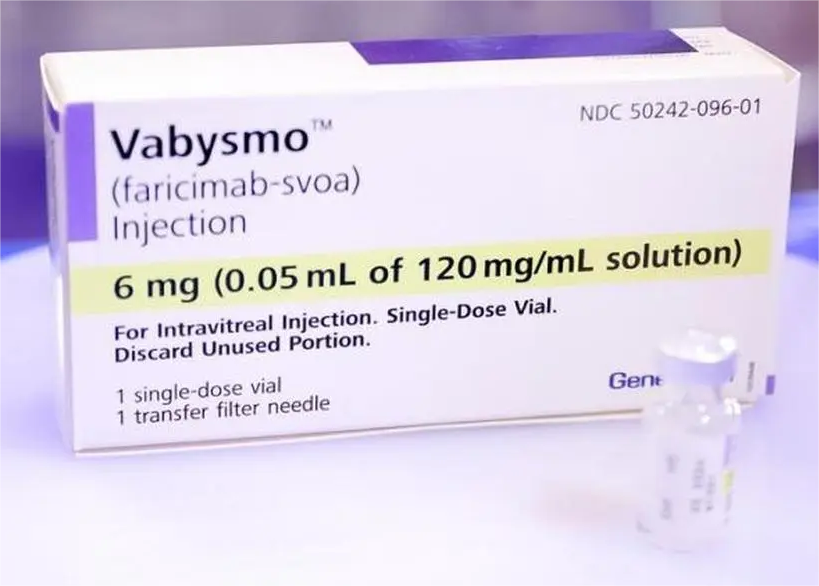Faricimab Injection.
Farximab is a humanized bispecific immunoglobulin G1 (IgG1) antibody against vascular endothelial growth factor (VEGF) and angiopoietin-2 (Ang-2), produced by Chinese hamster ovary (CHO) cells through DNA recombinant technology.
Excipients: histidine, 30% acetic acid, methionine, sodium chloride, sucrose and polysorbate 20
[Properties] Clear to opalescent, colorless to brown-yellow solution.
[Indications] This product is a bispecific VEGF and Ang-2 inhibitor used to treat:
● Diabetic macular edema (DME)
● Neovascular (wet) age-related macular degeneration (nAMD)
[Specifications] 0.05ml (6mg)/bottle
[Usage and Dosage] General Administration Information
Intravitreal injection. This product should be administered by a qualified physician. Each bottle of medicine is for monocular treatment only.
Diabetic macular edema (DME)
It is recommended to administer the drug according to one of the following two dosing regimens:
1. 6 mg (equivalent to 0.05 ml) once, administered by intravitreal injection once every 4 weeks (approximately once every 28 days ± 7 days, once a month), for at least 4 doses. After completing at least 4 doses, if the edema subsides as determined by the macular central retinal thickness (CST) measured by optical coherence tomography (OCT), the dosing interval can be adjusted based on the results of CST and visual acuity assessment. If the condition is stable, the dosing interval can be gradually extended to once every 16 weeks, with each extension not exceeding 4 weeks; if the condition worsens, the dosing interval should be shortened, with each shortening not exceeding 8 weeks.
2. 6 mg (equivalent to 0.05 ml) once, administered by intravitreal injection once every 4 weeks (approximately once every 28 days ± 7 days, once a month), for 6 consecutive doses, followed by a 6 mg dose once every 8 weeks (2 months).
Note: Although no improvement in efficacy was observed in most patients with every 4-week dosing compared to every 8-week dosing, some patients may need to continue treatment at the original every 4-week (once a month) dosing schedule after completing the first 4 doses. Patients should be evaluated regularly.
Neovascular (wet) age-related macular degeneration (nAMD)
The recommended dose of this product is 6 mg (equivalent to 0.05 ml) once, administered once every 4 weeks (approximately every 28±7 days, once a month) by intravitreal injection for 4 consecutive doses. Disease activity was assessed based on optical coherence tomography (OCT) and visual acuity at 8 and 12 weeks after completion of the fourth dose to determine continued dosing according to one of the following three dosing schedules: 1) Weeks 28 and 44 (i.e., dosing every 16 weeks); 2) Weeks 24, 36, and 48 (i.e., dosing every 12 weeks); or 3) Weeks 20, 28, 36, and 44 (i.e., dosing every 8 weeks).
Share:
Products
Our offers
Health Classification
Let us work together to protect precious health