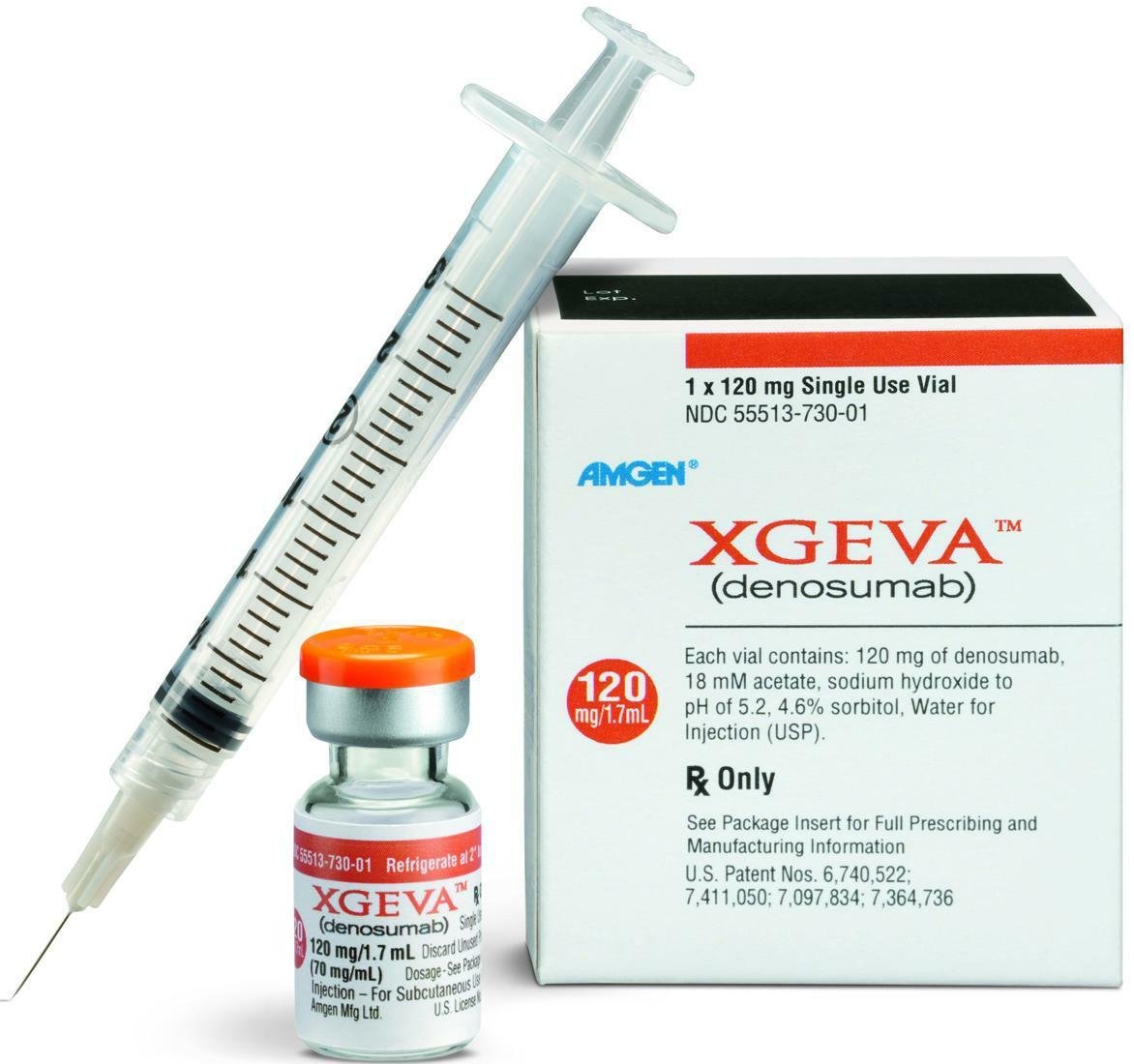
Denosumab Injection
Denosumab (also known as AMC-162, trade name Prolia) is a bone resorption inhibitor with a unique mechanism of action. It specifically targets the receptor activator of NF-kB ligand (RANKL), inhibits osteoclast activation and development, reduces bone resorption, and increases bone density. On May 28, 2010, the European Commission approved this product for the treatment of osteoporosis in postmenopausal women and hormone suppression-related bone loss in prostate cancer patients. It is also used for patients who are ineffective or intolerant of other current treatments to reduce the risk of fractures in patients. This product was first approved in 27 EU member states as well as Norway, Iceland, and Liechtenstein. In June of the same year, this product was approved by the FDA for marketing.
Indications
This product is mainly used for the following:
1. For the treatment of osteoporosis in menopausal women (with high-risk fractures).
2. For the treatment of bone loss in prostate cancer patients (caused by androgen blockade therapy).
3. For the treatment of bone loss in breast cancer patients (caused by aromatization inhibitor therapy).
Share:
Products
Our offers
Health Classification
Let us work together to protect precious health