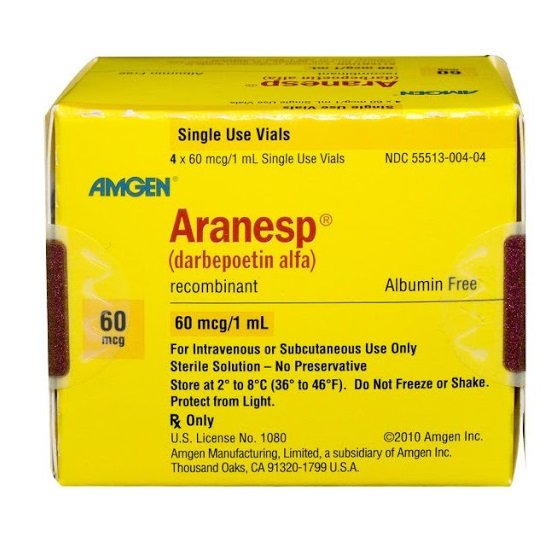
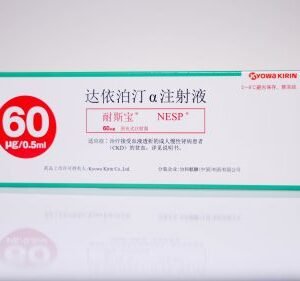
Darbepoetin alfa Injection.
NESP® is a long-acting erythropoietin preparation. Through genetic engineering and glycoprotein engineering technology, the structure of traditional erythropoietin has been modified to improve the plasma half-life and biological activity. In June 2020, it obtained the import drug registration certificate from the National Medical Products Administration and is the first long-acting EPO-α preparation approved in my country. NESP®/NESP® is currently used in more than 70 countries around the world, and has replaced short-acting erythropoietin EPO for the treatment of renal anemia in most developed countries and regions.
Brand:
injection
Categories: B blood circulation
Share:
Products
Our offers
Delivery – when you want and anywhere
A huge selection of best products
Health Classification
Let us work together to protect precious health