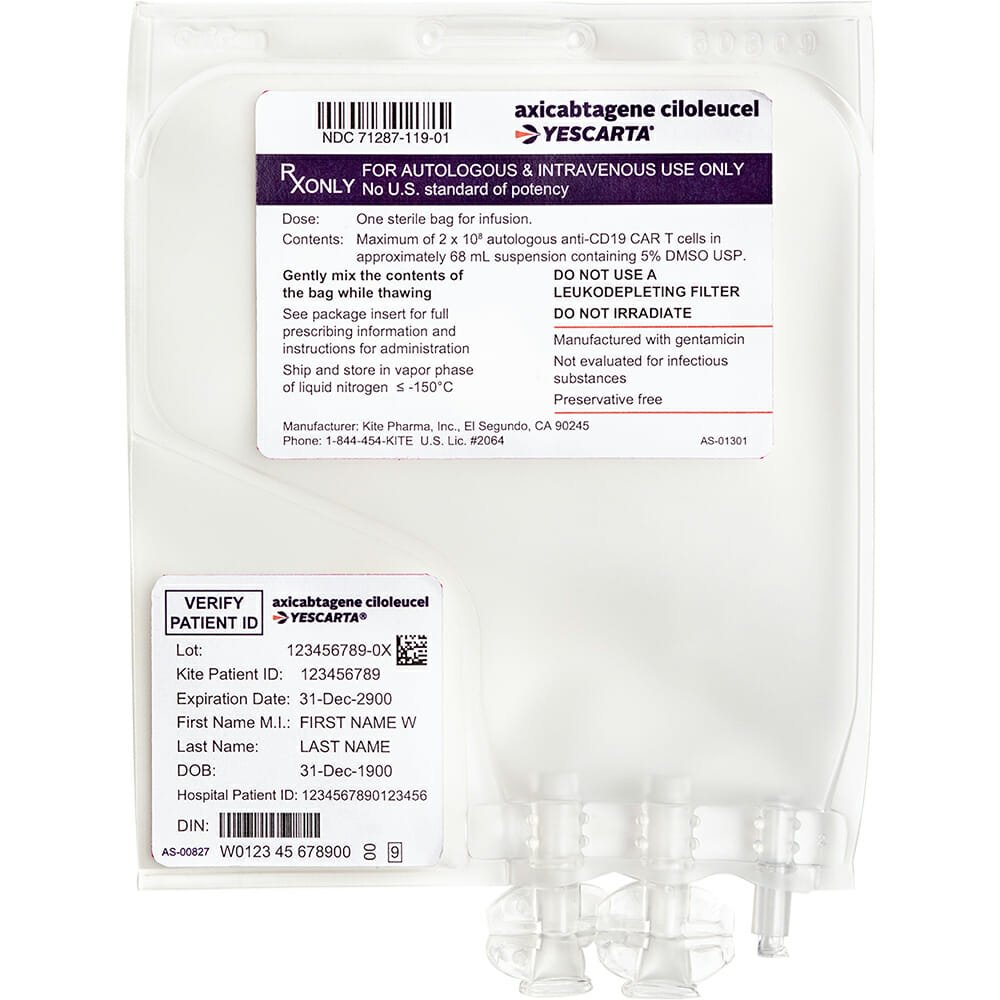
Axicabtagene Ciloleucel Injection,阿基仑赛注射液
Effect and efficacy: This product is a genetically modified chimeric antigen receptor autologous T (CAR-T) cell targeting human CD19, used to treat adult patients with relapsed or refractory large B-cell lymphoma after receiving two or more lines of systemic treatment, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), primary mediastinal large B-cell lymphoma (PMBCL), high-grade B-cell lymphoma, and DLBCL transformed from follicular lymphoma; Akilencel injection is a genetically modified autologous T cell immunotherapy targeting CD19 that can bind to tumor cells and normal B cells expressing CD19. Studies have shown that when anti-CD19 CAR-T cells bind to target cells expressing CD19, the CD28 and CD3-zeta co-stimulatory domains activate downstream cascade signals, leading to T cell activation, proliferation, acquisition of effector function, and secretion of inflammatory cytokines and chemokines. This series of events leads to the killing of cells expressing CD19.
Dosage and Administration:
Cyclophosphamide 500 mg/m2 and fludarabine 30 mg/m2 are intravenously infused for lymphodepleting chemotherapy on days 5, 4, and 3 before infusion of this product. Pre-infusion medication: About 1 hour before infusion of this product, take acetaminophen 500-1000 mg orally and diphenhydramine 12.5-25 mg orally or intravenously. Preparation for infusion of this product requires coordination of the time of reconstitution and infusion of this product; reconstitute this product in a water bath at about 37°C until there are no ice crystals visible in the product bag. Gently mix the contents of the bag to disperse aggregated cells. If there are still visible cell clumps, continue to gently mix the contents of the bag and use gentle manual methods to disperse small cell clumps. Do not wash, centrifuge and/or resuspend this product before infusion. Once reconstituted, infuse as soon as possible. This product can be stored at 20°C~25°C for 3 hours; after infusion, monitor for at least 10 days in a medical institution that has been evaluated and trained to observe cytokine release syndrome (CRS) symptoms and neurological toxicity. The same drug produced by different manufacturers may have inconsistent instructions. The above content is referenced from the instructions for Akilencel injection (National Medicine Standard S20210019). If you find inconsistencies in the instructions before taking the drug, please consult a doctor or pharmacist in time.
Drug contraindications:
Allergy to this product is prohibited. Use with caution during lactation. Use with caution in patients with liver and kidney dysfunction. Use with caution in children. Use with caution during pregnancy. Use with caution when driving.
Related dosage forms:
Injection
Share:
Products
Our offers
Health Classification
Let us work together to protect precious health